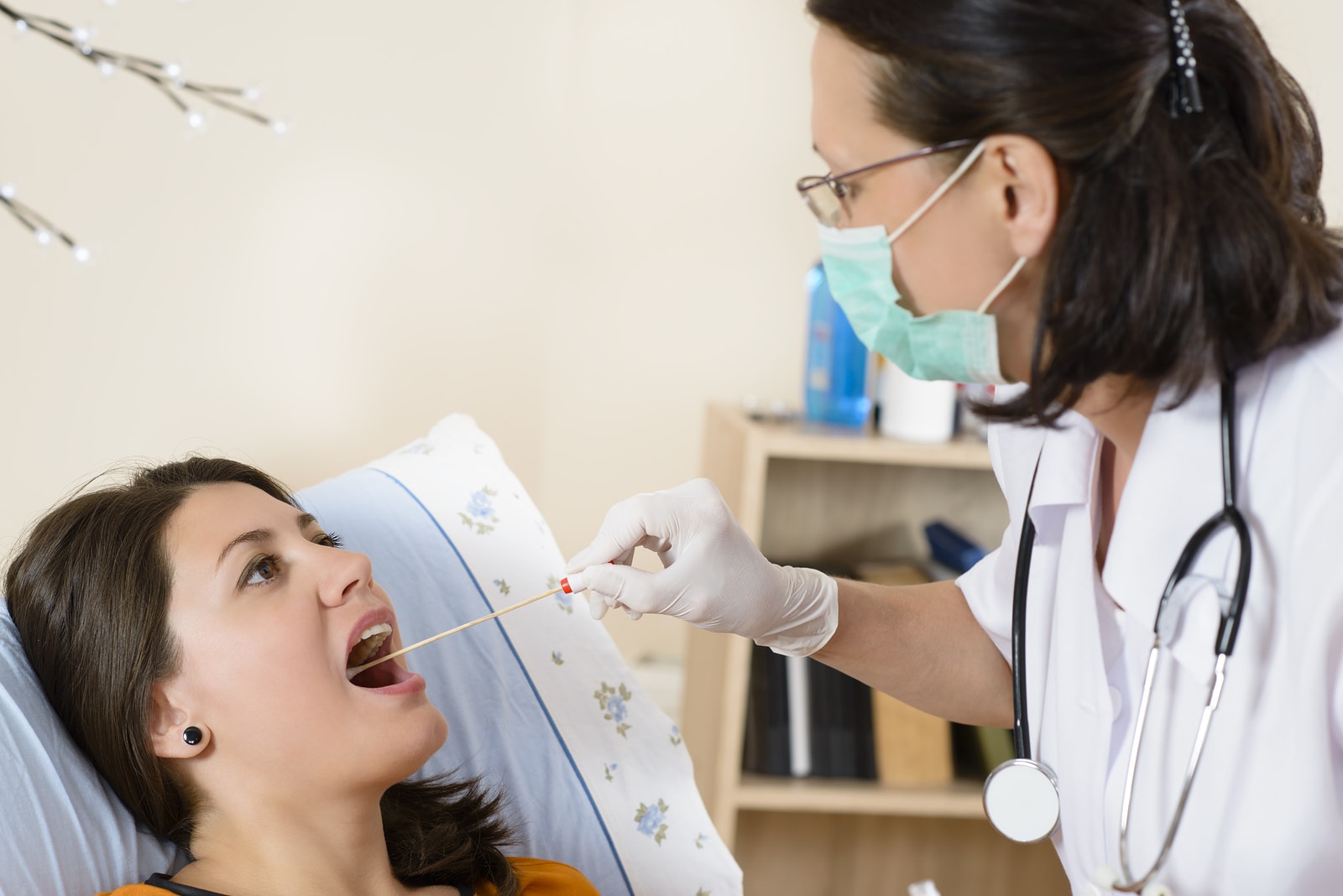
A new test being developed by researchers that uses a drop of saliva and takes just 10 minutes to detect cancer represents a possible diagnostic breakthrough in early disease detection.
Using a technique described as a “liquid biopsy,” the test detects the DNA of tumors that are circulating in body fluids.
University of California, Los Angeles Professor of Oncology David Wong said the test is 100% accurate. Wong said the test isn’t complex and could be successfully conducted by a dentist or pharmacist – or even by someone at home.
“Early detection is crucial. Any time you gain in finding out that someone has a life-threatening cancer, the sooner the better,” Wong said.
Wong presented his team’s findings at the American Association for the Advancement of Science (AAAS) annual meeting in Washington, D.C. this month. He showcased an electric field-induced release and measurement (EFIRM) device that can detect saliva biomarkers for non-small cell lung cancer (NSCLC). The test has been tried successfully thus far on lung cancer patients only.
How it works
“After lung cancer, any type of human cancer can be detected, as circulating tumor DNA is universal,” Wong said. “Our finding that the [cell-free circulating tumor DNA] (ctDNA) comes into saliva and that EFIRM is the technology that can detect the ctDNA with near-perfect sensitivity is the perfect platform.”
You Might Also Enjoy: Injectable Liquid Illuminates Cancer Cells, May Help Surgeons Locate and Remove Tumors
At present, the only way cancer can be detected via blood tests is if a biopsy had already been performed and the tumor sequenced, which gives doctors a genetic signature to seek. That procedure, however, may be subject to false positives and can’t be used for initial tests, although it can monitor the spread of cancer.
Not only is this new test fast, convenient and easy to perform, but it will only cost about $25. Compare that to a biopsy, which can cost $2,000 or more in some cases and may take several days before results are available. In comparison, a mutation analysis typically takes two weeks and costs $450.
Wong did note that his test “should be viewed as a screening tool or for risk assessment only in symptomatic patients and that it is not meant to replace biopsy.”
The test can also be combined with other diagnostic methods. The report cited the example of a chest x-ray with a suspicious lump; the saliva test could be used to determine if the lump is cancerous.
The Future
Clinical trials in the United States are anticipated by the end of 2016, with potential approval by the Food and Drug Administration in 2018.
Saliva test prototypes will be tested in Europe and China in 2016, Huffington Post UK reported. The test could be for sale to the public in the United Kingdom by 2020.
In time, the test might become more versatile, Wong told The Telegraph. “Down the road it might be possible to test for multiple cancers at the same time.”