Opioid abuse in the United States has been called an epidemic. A crisis.
According to the Centers for Disease Control and Prevention (CDC), Opioid-related deaths surpassed cocaine in the early 2000s, and it’s been rising steadily ever since. By 2004, more people were dying from opioid abuse than from cocaine and heroin abuse combined. Deaths associated with the misuse of prescription opioids reached 19,000 in 2014. Together, heroin and opioid painkillers have led to nearly 29,000 fatal overdoses.
More than half a million people in the United States currently use heroin.
Two-thirds of those people used prescription opioids before turning to heroin. Generally, most opioid abusers eventually make the switch to heroin because it sells for far less than illegally-sold prescription pills.
New Warning Labels
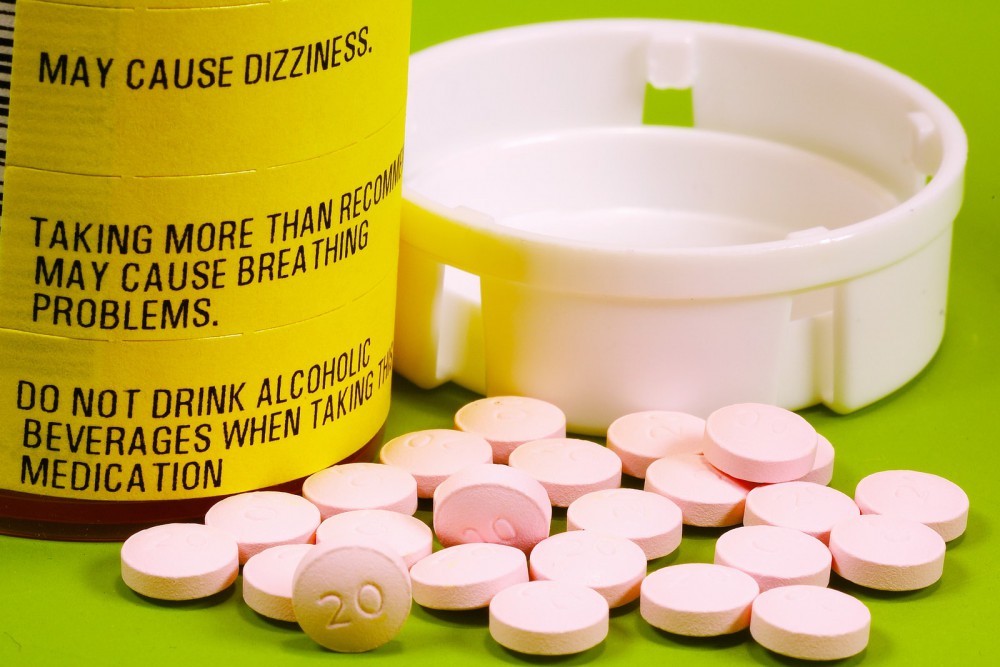
In February, the U.S. Food and Drug Administration (FDA) announced they were going to take measures to try to stem opioid abuse. They would start simply – officials decided to take a closer look at how opioids were reviewed and labelled. Less than two months later, the FDA has followed through on that decision.
“We’re at a time when the unfathomable tragedies resulting from addiction, overdose and death have become one of the most urgent and devastating public health crises facing our country,” said FDA Commissioner Dr. Robert Califf, M.D. “I can’t stress enough how critical it is for prescribers to have the most current information.”
From now on, all immediate-release opioid painkillers (almost 200 drugs in total) will come with a boxed warning, the strongest of the FDA’s warning labels. This act is part of a greater and more thorough campaign against opioid abuse, which includes drugs like Percocet and Vicodin. Those two medications, in particular, are among the most frequently prescribed in the country; 9 of every 10 opioid prescriptions are for one or the other.
You Might Also Enjoy: Walgreens to Sell Naloxone, Heroin Overdose Remedy, Over-the-Counter
This isn’t the first time the FDA has stepped forward and bolstered warnings on commonly-prescribed medications. Three years ago, OxyContin was one of the recipients of a similar boxed warning. Unlike Percocet and Vicodin, OxyContin is a slow-release drug, but now they will all share a similar label, one that warns about the risks of abuse, addiction, overdose and possible death.
“The main driver of our opioid epidemic is addiction, and the immediate-release products are just as addictive,” Dr. Andrew Kolodny, addiction therapist and founder of Physicians for Responsible Opioid Prescribing, told the Associated Press. “That’s why they should be prescribed more cautiously.”
Discouraging Opioid Abuse
Along with the warnings about abuse and addiction, the new labels will specify that the medications should be used, exclusively, for pain that cannot be managed with other drugs or therapies. It’s a last resort.
“This new indication, once finalized, will remind prescribers that immediate-release opioids are also powerful drugs with important safety concerns,” said Dr. Doug Throckmorton, a deputy director in the FDA’s drug center.
The FDA’s campaign coincides with similar efforts from the CDC, who recently implemented national prescribing guidelines for opioids. These guidelines advise primary care doctors to turn to opioid prescriptions only after considering a myriad of other options, like physical therapy, over-the-counter treatments, counseling, and other methods.









